Background
Outcomes in relapsed/refractory (R/R) peripheral T-cell lymphoma (PTCL) continue to be suboptimal. In the last decade, several novel agents have been approved; however, their impact on survival remains to be defined. In this multi-center retrospective analysis, we assessed survival following initial progression with respect to novel agent exposure with and without the use of stem cell transplant (SCT).
Methods
Patients with PTCL diagnosed between 1998-2021 at New York Presbyterian Hospitals, Columbia and Cornell, were included after IRB approval. Medical records were reviewed for baseline characteristics and treatment parameters. In the second line setting, each patient fell into one of four categories: 1. novel agent containing regimen only ( novel-containing only), 2. novel agent containing regimen with SCT at end of second line ( novel + SCT), 3. chemotherapy alone ( chemo-only), and 4. chemotherapy with SCT at end of second line ( chemo + SCT). Event-free survival (EFS) was calculated as time from 1 st progression to 2 nd progression, re-treatment, or death. Subsequent OS (sOS) was calculated from date of first progression to death or last follow-up. Kaplan-Meier method was used to estimate survival probability. Survival difference was tested by log-rank and Cox regression analysis.
Results
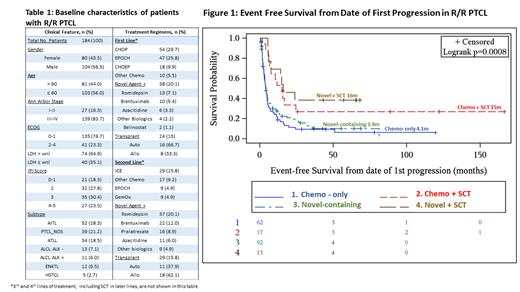
A total of 348 patients with PTCL were identified, of which 189 had R/R disease and 184 had sufficient data for analysis. The median follow-up was 62.5m. Baseline characteristics are shown in Table 1. Most common PTCL subtypes were AITL (N=52, 28.3%), PTCL-NOS (N=39, 21.2%), ATLL (N=34, 18.5%), and ALCL (ALK- = 13, ALK+ = 11, n=24, 13%). Median age at diagnosis was 58 years. Median EFS and sOS from date of initial progression for the entire cohort was 5.0m and 25.1m, respectively. In the second-line setting, 92 (50%) received a novel-containing only, 62 (34%) patients received chemo-only, 17 (9%) received chemo + SCT, and 13 (7%) received novel + SCT. Median EFS from first progression among patients who received second-line novel + SCT was 16.0m (95% CI 6.0 - NR), chemo + SCT 15.0m (95% CI 6.2 - 24.5), novel-containing only 3.9m (95% CI 3.5 - 5.3), and chemo-only 4.1m (95% CI 2.9 - 5.1) as shown in Figure 1. Two-year EFS favored novel + SCT at 38.5m (95% CI 12.0 - 64.9) and chemo + SCT 34.0m (95% CI 10.8 - 57.1), compared to novel-containing only at 18.3m (95% CI 10.1 - 26.6) and chemo-only 11.3m (95% CI 3.0 - 19.6), with p < 0.001 across all groups. Median sOS among patients who received second-line novel + SCT was 70.7m (95% CI 16.0 - NR), chemo + SCT not reached (95% CI 15.0 - NR), novel-containing only 23.3m (95% CI 10.5 - NR), and chemo-only 16.7m (95% CI 11.7 - 27.0). Two-year sOS favored novel + SCT at 74.6m (95% CI 49.7 - 99.5) and chemo + SCT 70.4m (95% CI 48.5 - 92.2) compared to novel-containing only at 49.4m (95% CI 37.9 -61.0) and chemo-only 43.4m (95% CI 30.0 - 56.9), with p =0.014 across all groups.
Conclusion
This multi-center study represents one of the largest retrospective R/R PTCL cohorts in the modern era with sOS of 25.1m, which compares favorably to historical data collected a decade ago reporting a sOS of 5.5m in R/R patients receiving chemo-only (Mak 2013), however, compares similarly to more recent data from the COMPLETE study (Lansigan 2019). This improvement in sOS may reflect availability of more effective therapies for sequencing in the novel agent era. Our data suggests that SCT in second-line setting is associated with improved EFS and sOS for both those treated with novel agent containing regimens and chemotherapy. Differences are more prominent for two-year EFS and sOS, favoring SCT arms and novel agents. However, the retrospective nature of our data limits definitive conclusion of benefit derived from SCT in the second-line setting, which underscores the need for prospective studies in this area.
*This work has been funded ASH RTAF
Disclosures
Seshadri:Kite: Consultancy; BeiGene: Consultancy; Roche: Research Funding; Eli Lilly: Research Funding. Martin:AbbVie, AstraZeneca, Beigene, Epizyme, Genentech, Gilead, Janssen, Pepromene, Daiichi Sankyo: Consultancy. Leonard:National Cancer Institute, Leukemia and Lymphoma Society, Genentech, Epizyme, Janssen: Research Funding; AbbVie, AstraZeneca, Astellas, Bayer, BeiGene, BMS, Calithera, Constellation, Eisai, Epizyme, GenMab, Grail, Incyte, Janssen, Karyopharm, Lilly, Merck, Mustang Bio, Pfizer, Roche/Genentech, Seagen, Second Genome, Sutro: Consultancy. Pro:Bio Secura: Honoraria; Seattle genetics: Honoraria. Amengual:Incyte: Consultancy; Epizyme: Honoraria; AstraZeneca: Consultancy. Ruan:Secura Bio: Consultancy; Daiichi Sankyo: Research Funding; Genentech: Research Funding; BMS: Research Funding; AstraZeneca: Consultancy, Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal